FDA's Interpretation Of The “Deemed To Be A License” Provision Of The Biologics Price Competition An | Contract Pharma

Meeting FDA Guidance recommendations for replication-competent virus and insertional oncogenesis testing: Molecular Therapy - Methods & Clinical Development

Summary of Guidance for Minimizing the Impact of COVID-19 on Individual Persons, Communities, and Health Care Systems — United States, August 2022 | MMWR
FDA Emergency Use Authorization: A Brief History From 9/11 to COVID-19 - Food and Drug Law Institute (FDLI)
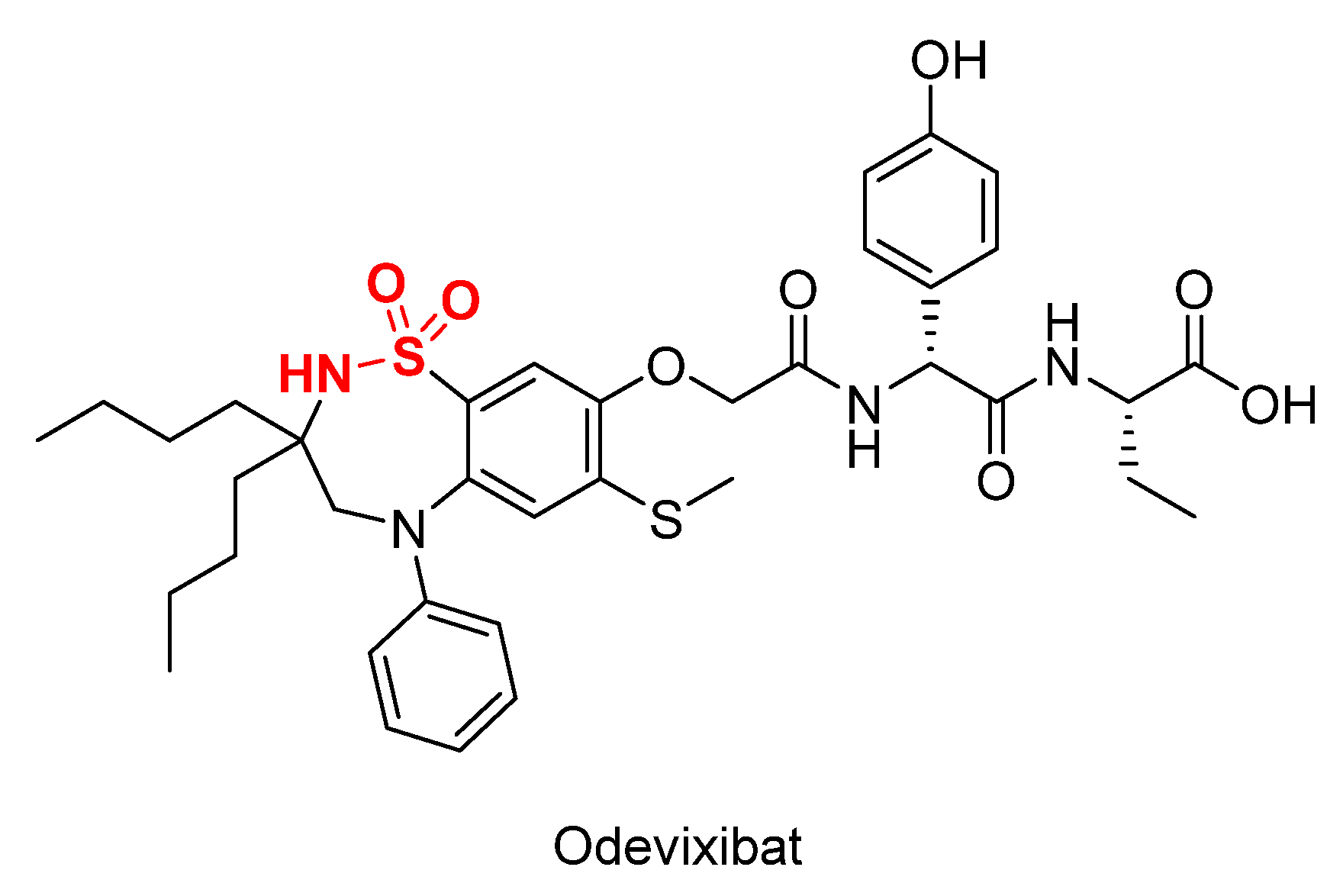
Pharmaceutics | Free Full-Text | FDA-Approved Small Molecules in 2022: Clinical Uses and Their Synthesis
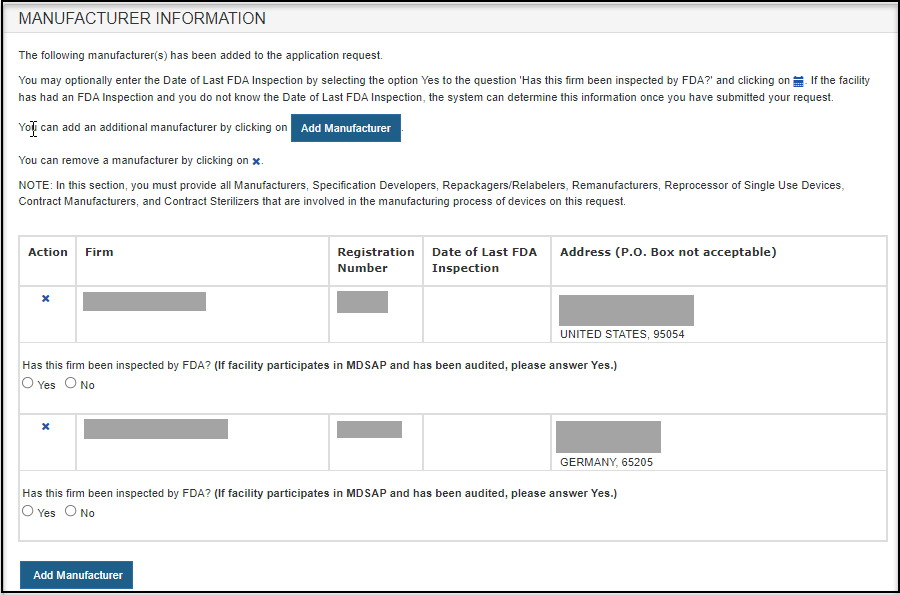
FDA Food Facility Registration is required under laws created by both the Bioterrorism Act of 2003 and Food Safety Modernization

The FDA Verification Portal 🔎 Check if an establishment is licensed and/or verify if a health product is registered with the Food and Drug... | By Food and Drug Administration Philippines